Endobronchial Valve Improves Breathing (Dyspnea)
Zephyr Endobronchial Valve Improves Breathing Compared With Standard Care
Background
Shortness of breath, known as the medical word – dyspnea, is the main symptom of COPD. It typically limits physical activities and is frequently disabling for those with advanced disease. Relief of shortness of breath is one the key goals or treating COPD.
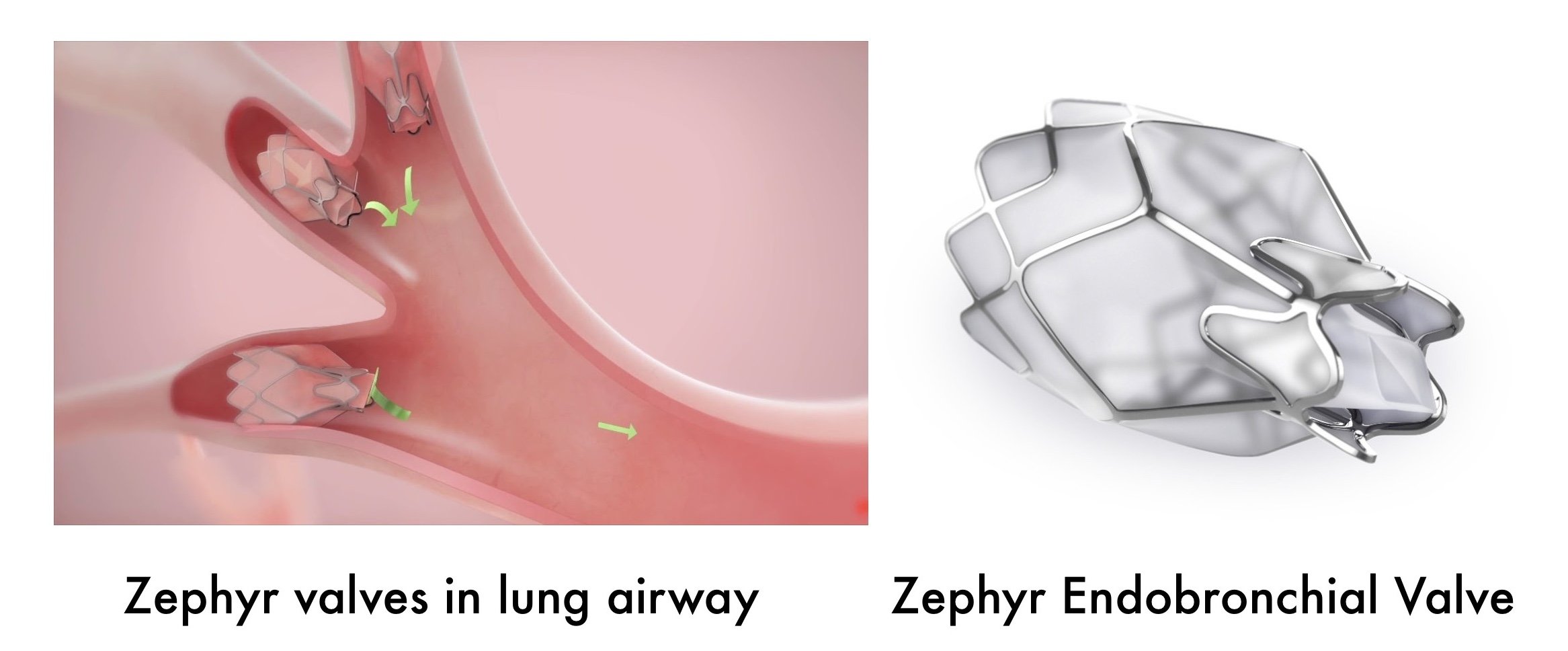
In June 2018, the US Food and Drug Administration approved the Zephyr Endobrochial Valve System as an implantable bronchial valve used to reduce over inflation of the lungs due to severe emphysema. The device consists of a one-way silicone duckbill valve attached to a nickel-titanium (Nitinol) self-expanding retainer that is covered with a silicone membrane.
Zephyr Endobronchial Valve system
Study
Dr. Mark Dransfield and colleagues reported on the effect of Zephyr endobronchial valves on breathlessness (dyspnea) at one year in the LIBERATE study.
In brief, the study compared outcomes in 190 subjects with severe emphysema and hyperinflation (too much air in the lungs). Subjects were assigned by chance (randomized) to receive one or more Zephyr endobronchial valves (128 subjects) or standard of care (62 subjects). Various questionnaires were used to measure shortness of breath at the beginning of the study and one year later.
The report was published in the July 2020 issue of the Annals of the American Thoracic Society (volume 17; pages 829-838).
Results
Overall, subjects who received valve treatment had significant and meaningful improvements is distance walked in 6 minutes, shortness of breath on the Transition Dyspnea Index (TDI), and quality of life on the St. George’s Respiratory Questionnaire (SGRQ) compared with those who received standard of care (SoC) (Figure below).
Responders (percent of patients) who improved by the minimal importance difference
Subjects also completed a daily dairy which showed significantly more days when their emphysema symptoms were better than at the start of the study with Zephyr valves (206 days) compared with standard of care (102 days).
Conclusions
Those with severe hyperinflated emphysema who received placement of Zephyr valves experienced improvements in breathlessness, activity, and psychosocial aspects for one year compared with standard of care.
My Comments
The benefits of Zephyr valve placement – relief of shortness of breath and improved quality of life – are impressive. It is important to recognize that this study was performed in a select group of individuals with COPD:
emphysema type
hyperinflation or “too much air in the lungs” as measured by breathing tests
no evidence of collateral ventilation between lobes of the lungs
How do you know if you qualify?
It is reasonable to start with an appointment with a pulmonologist to find out if you meet the first two conditions – emphysema and hyperinflation. Then you will need to be referred to a hospital or medical center that is placing Zephyr bronchoscopic valves to find out if you meet the third condition – no collateral ventilation. The article lists the different sites where the study was performed – called The LIBERATE Study Group.